Platinum(ii)–chloroquine complexes are antimalarial agents against blood and liver stages by impairing mitochondrial function†
Abstract
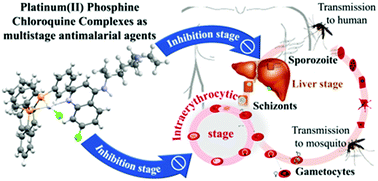
Chloroquine is an antimalarial agent with strong activity against the blood stage of Plasmodium infection, but with low activity against the parasite's liver stage. In addition, the resistance to chloroquine limits its clinical use. The discovery of new molecules possessing multistage activity and overcoming drug resistance is needed. One possible strategy to achieve this lies in combining antimalarial quinolones with the pharmacological effects of transition metals. We investigated the antimalarial activity of four platinum(II) complexes composed of chloroquine and phosphine ligands, denoted as WV-90, WV-92, WV-93 and WV-94. In comparison with chloroquine, the complexes were less potent against the chloroquine-sensitive 3D7 strain but they were as active as chloroquine in inhibiting the chloroquine-resistant W2 strain of P. falciparum. Regarding selectivity, the complexes WV-90 and WV-93 displayed higher indexes. Unlike chloroquine, the complexes act as irreversible parasiticidal agents against trophozoites and the WV-93 complex displayed activity against the hepatic stage of P. berghei. The in vivo suppression activity against P. berghei in the Peters 4 day test displayed by the complexes was similar to that of chloroquine. However, the efficacy in an established P. berghei infection in the Thompson test was superior for the WV-93 complex compared to chloroquine. The complexes’ antimalarial mechanism of action is initiated by inhibiting the hemozoin formation. While chloroquine efficiently inhibits hemozoin, parasites treated with the platinum complexes display residual hemozoin crystals. This is explained since the interaction of the platinum complexes with ferriprotoporphyrin is weaker than that of chloroquine. However, the complexes caused a loss of mitochondrial integrity and subsequent reduction in mitochondrial activity, and their effects on mitochondria were more pronounced than those in the chloroquine-treated parasites. The dual effect of the platinum complexes may explain their activity against the hemozoin-lacking parasites (hepatic stage), where chloroquine has no activity. Our findings indicate that the platinum(II)–chloroquine complexes are multifunctional antimalarial compounds and reinforce the importance of metal complexes in antimalarial drug discovery.


 Please wait while we load your content...
Please wait while we load your content...