Novel bipharmacophoric inhibitors of the cholinesterases with affinity to the muscarinic receptors M1 and M2†‡
Abstract
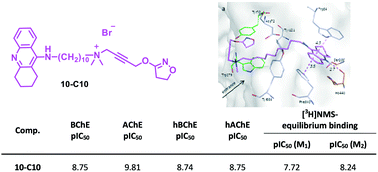
A set of hybrid compounds composed of the fragment of allosteric modulators of the muscarinic receptor, i.e. W84 and naphmethonium, and the well-known AChE inhibitor tacrine on the one hand, and the skeletons of the orthosteric muscarinic agonists, iperoxo and isox, on the other hand, were synthesized. The two molecular moieties were connected via a polymethylene linker of varying length. These bipharmacophoric compounds were investigated for inhibition of AChE (from electric eel) and BChE (from equine serum) as well as human ChEs in vitro and compared to previously synthesized dimeric inhibitors. Among the studied hybrids, compound 10-C10, characterized by a 10 carbon alkylene linker connecting tacrine and iperoxo, proved to be the most potent inhibitor with the highest pIC50 values of 9.81 (AChE from electric eel) and 8.75 (BChE from equine serum). Docking experiments with compounds 10-C10, 7b-C10, and 7a-C10 helped to interpret the experimental inhibitory power against AChE, which is affected by the nature of the allosteric molecular moiety, with the tacrine-containing hybrid being much more active than the naphthalimido- and phthalimido-containing analogs. Furthermore, the most active AChE inhibitors were found to have affinity to M1 and M2 muscarinic receptors. Since 10-C10 showed almost no cytotoxicity, it emerged as a promising lead structure for the development of an anti-Alzheimer drug.


 Please wait while we load your content...
Please wait while we load your content...