Design, facile synthesis and anthelmintic activity of new O-substituted 6-methoxybenzothiazole-2-carbamates. Part II†
Abstract
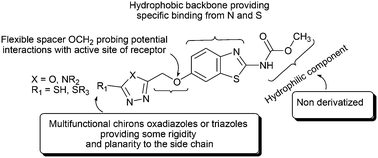
In the framework of pursuing the design and synthesis of a new series of substituted 6-methoxybenzothiazole-2-carbamates as potential anthelmintics, and as a continuation of the expended efforts in part I, we have set out to develop novel compounds with enhanced anthelmintic activity by blocking the 6-position of benzothiazole with side chains of different polarities. Guided by the findings in part I, and reporting the paramphistomicidal activity of oxadiazoline derivatives V and VI, we aimed to synthesize target benzothiazoles designed to comprise some planar heterocyclic ring systems, namely, 1,3,4-oxadiazoles and 1,2,4-triazoles, bearing a variety of hydrophobic and hydrophilic components. The synthesis of the desired compounds was primarily achieved by cyclization of 6-acetohydrazide, 1. The in vitro paramphistomicidal activity of all synthesized carbamates was evaluated. Four synthesized carbamates exhibited notable activity. Compound 24, methyl 6-[(5-(4-bromophenacylsulfanyl)-[1,3,4]-oxadiazol-2-yl)methoxy]benzothiazole-2-carbamate, displayed an equipotent effect to the reference drug oxyclozanide at a concentration of 80 μg mL−1; compounds 9, 10 and 23 showed high orders of anthelmintic effect. A structural computational study on the polar nature and hydrophilic–lipophilic properties of the synthesized carbamates was undertaken to discuss their structure–activity relationship (SAR). Besides, pharmacophore mapping was performed using eight active compounds as a training set. The generated pharmacophore model revealed five common features and was validated using fenbendazole, triclabendazole and triclabendazole sulfoxide.


 Please wait while we load your content...
Please wait while we load your content...