Synthesis and biological evaluation of 8-hydroxy-2,7-naphthyridin-2-ium salts as novel inhibitors of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE)†‡
Abstract
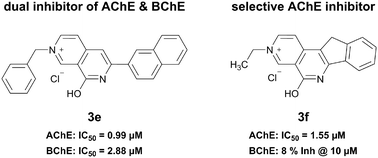
By analogy with the natural product chelerythrine, which has been identified as an inhibitor of both acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), we prepared a small series of 8-hydroxy-2,7-naphthyridin-2-ium salts. Spectroscopic analyses allowed us to elucidate the zwitterionic nature of 2,7-naphthyridin-1(7H)-ones, the neutral state of 8-hydroxy-2,7-naphthyridin-2-ium salts. Among the tested compounds, we identified dual inhibitors of AChE and BChE as well as an inhibitor showing a preferential inhibition of AChE over BChE. By in vitro characterization in combination with docking studies, we were able to identify structural features that influence the biological activity of 8-hydroxy-2,7-naphthyridin-2-ium salts.


 Please wait while we load your content...
Please wait while we load your content...