A scaffold merging approach to Hsp90 C-terminal inhibition: synthesis and evaluation of a chimeric library†‡
Abstract
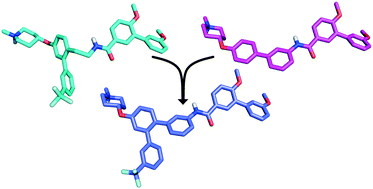
Inhibition of the Hsp90 C-terminus is an attractive therapeutic paradigm for the treatment of cancer, however the developmental space of C-terminal inhibitors is limited. It was hypothesized that the combination of two previously identified scaffolds into a single structure could provide a platform for which to probe the three-dimensional space within the Hsp90 C-terminal binding pocket. The resulting chimeric compounds displayed anti-proliferative activity at low micromolar concentrations and manifested inhibitory activity in an Hsp90-dependent rematuration assay. Initial structure–activity relationships suggest that this new scaffold binds Hsp90 in a conformation different from that of the parent compounds, and consequently, provides a new opportunity to develop more efficacious inhibitors of the Hsp90 C-terminal binding pocket.


 Please wait while we load your content...
Please wait while we load your content...