The Henipavirus V protein is a prevalently unfolded protein with a zinc-finger domain involved in binding to DDB1†
Abstract
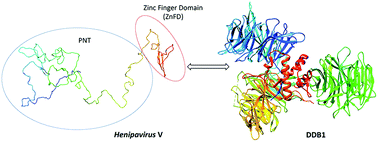
Henipaviruses are severe human pathogens within the Paramyxoviridae family. Beyond the P protein, the Henipavirus P gene also encodes the V protein which shares with P its N-terminal, intrinsically disordered region (PNT) and possesses a unique C-terminal domain predicted to be folded and to bind zinc (ZnFD). Henipavirus V proteins antagonize IFN signaling through PNT-mediated binding to STAT1, and several paramyxoviral V proteins promote STAT1 degradation through binding to DDB1. Structural and molecular information on Henipavirus V proteins is lacking, and their ability to interact with DDB1 has not been documented yet. We cloned the V genes from Nipah and Hendra viruses and purified the V proteins from E. coli and DDB1 from insect cells. Using analytical size-exclusion chromatography, CD and SAXS we characterized the V proteins and their domains. Using pull-down and MST we assessed their binding abilities towards DDB1. We show that PNT remains disordered also in the context of the V protein, while the ZnFD adopts a predominant β conformation. We also show that the V proteins interact with DDB1 predominantly via their ZnFD. This is the first experimental characterization of the Henipavirus V proteins and the first experimental evidence of their interaction with DDB1. The DDB1–ZnFD interaction constitutes a promising target for antiviral strategies. These studies provide a conceptual asset to design new antiviral strategies expected to reduce or abrogate the ability of these viruses to escape the innate immune response. They also contribute to illuminating the conformational behaviour of proteins encompassing large intrinsically disordered domains.


 Please wait while we load your content...
Please wait while we load your content...