Enhanced directional cell migration induced by vaccinia virus on a microfluidic-based multi-shear cell migration assay platform†
Abstract
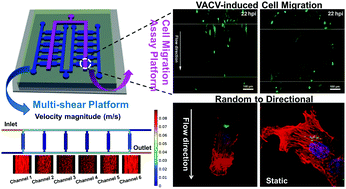
Virus-induced cell migration plays important roles in the direct and rapid spread of virus particles. As cell migration is also regulated by shear stress, it is necessary to explore the cell migration behavior influenced by multiple factors including a virus and shear stress. In this report, a three-layer microfluidic chip with symmetric channels was designed and fabricated to investigate vaccinia virus-induced cell migration in different shear stress environments. Regular “exclusion zones” without cell damage were formed by microvalves. The results showed that infected cells were more elongated and tended to migrate along the flow direction compared to the random cell migration under static conditions. Meanwhile, shear stress enhanced the natural directional persistence and accelerated the velocity of infected cell migration. Moreover, reduced peripheral lamellae and the axial lamella being confined to the flow direction were responsible for the increased directionality of cell migration under shear stress. Interestingly, in the presence of shear stress, the Golgi complex reoriented and relocated behind the nucleus and aligned to the flow direction in infected migratory cells accompanied by the rearrangement of the cytoskeleton. Our report reveals the cell migration behavior in the multi-environment of virus infection and shear stress based on the microfluidic cell migration assay platform. It helps us to deeply understand the interactions between the virus, host cells, and surrounding microenvironment.


 Please wait while we load your content...
Please wait while we load your content...