Correlation of focal adhesion assembly and disassembly with cell migration on nanotopography†
Abstract
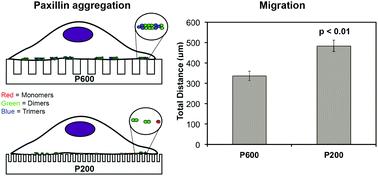
Selective cell adhesion is desirable to control cell growth and migration on biomedical implants. Mesenchymal cell migration is regulated through focal adhesions (FAs) and can be modulated by their microenvironment, including changes in surface topography. We use the Number and Molecular Brightness (N&B) imaging analysis to provide a unique perspective on FA assembly and disassembly. This imaging analysis generates a map of real-time fluctuations of protein monomers, dimers, and higher order aggregates of FA proteins, such as paxillin during assembly and disassembly. We show a dynamic view of how nanostructured surfaces (nanoline gratings or nanopillars) regulate single molecular dynamics. In particular, we report that the smallest nanopillars (100 nm spacing) gave rise to a low population of disassembling adhesion clusters of ∼2 paxillin proteins whereas the larger nanopillars (380 nm spacing) gave rise to a much larger population of larger disassembling clusters of ∼3–5 paxillin proteins. Cells were more motile on the smaller nanopillars (spaced 100–130 nm apart) compared to all other surfaces studied. Thus, physical nanotopography influences cell motility, adhesion size, and adhesion assembly and disassembly. We report for the first time, with single molecular detection, how nanotopography influences cell motility and protein reorganization in adhesions.
- This article is part of the themed collection: Integrative Biology Most Downloaded Articles


 Please wait while we load your content...
Please wait while we load your content...