Organocatalytic oxidation of substituted anilines to azoxybenzenes and nitro compounds: mechanistic studies excluding the involvement of a dioxirane intermediate†
Abstract
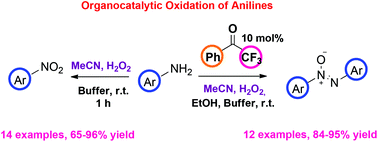
An organocatalytic and environmentally friendly approach for the selective oxidation of substituted anilines was developed. Utilizing a 2,2,2-trifluoroacetophenone-mediated oxidation process, substituted anilines can be transformed into azoxybenzenes, while a simple treatment with MeCN and H2O2 leads to the corresponding nitro compounds, providing user-friendly protocols that can be easily scaled up. Various substitution patterns and functional groups were tolerated leading to products in high to excellent yields. Mechanistic studies utilizing HRMS provide clear evidence for the distinct mechanistic intermediates that are involved. This study constitutes an indirect proof excluding the involvement of a dioxirane intermediate in the green organocatalytic oxidation, utilizing 2,2,2-trifluoroacetophenone as the catalyst.
- This article is part of the themed collection: 2017 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...