Biocatalytic synthesis of chiral cyclic γ-oxoesters by sequential C–H hydroxylation, alcohol oxidation and alkene reduction†
Abstract
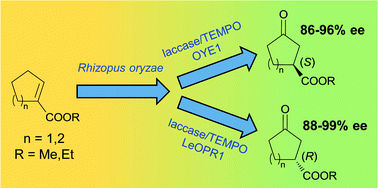
A three-step biocatalytic procedure is described for the conversion of methyl and ethyl cyclopentene- and cyclohexenecarboxylates into both the enantiomers of the corresponding chiral 3-oxoesters, which are useful building blocks for the synthesis of active pharmaceutical ingredients. The allylic hydroxylation of the starting cycloalkenecarboxylates is carried out by using R. oryzae resting cells entrapped in alginate beads, in acetate buffer solution at 25 °C. The oxidation of the intermediate allylic alcohols to unsaturated ketones, performed by the laccase/TEMPO system, and the ene-reductase mediated hydrogenation of the alkene bond were carried out in the same reaction vessel in a sequential mode at 30 °C. Being entirely biocatalytic, our multistep procedure provides considerable advantages in terms of selectivity and environmental impact over reported chemical methods.


 Please wait while we load your content...
Please wait while we load your content...