A practical synthesis of 2,3-dihydro-1,5-benzothiazepines†
Abstract
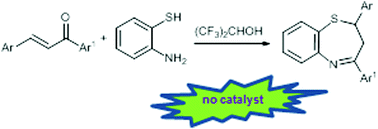
2,3-Dihydro-1,5-benzothiazepines have been obtained through a domino process involving a Michael addition of 2-aminothiophenols to chalcones, followed by in situ cyclization. Up to 98% chemical yields have been obtained at room temperature under essentially neutral conditions by using hexafluoro-2-propanol as an efficient medium.


 Please wait while we load your content...
Please wait while we load your content...