Activation of (salen)CoI complex by phosphorane for carbon dioxide transformation at ambient temperature and pressure†
Abstract
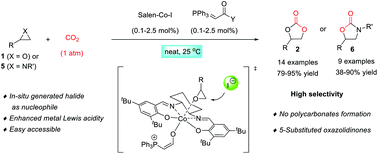
We report the activation of (salen)CoI complex 3g by a phosphorane to form a bifunctional catalyst for the reaction of carbon dioxide with terminal epoxides or aziridines at ambient temperature and 1 bar carbon dioxide pressure. Only 1.0 mol% of both (salen)CoI 3g and phosphorane 4d are required for cyclic carbonate synthesis, and the catalyst loading could even be lowered down to 0.1 mol%. Under these conditions, no polycarbonate formation is detected by NMR analysis. It is proposed that the high efficiency originates from the activation of (salen)CoI by a phosphorane to form a phosphorane–salen Co(III) complex with enhanced Lewis acidity for the electrophilic activation while generating an iodide anion as a Lewis base co-catalyst to facilitate the ring-opening of epoxides. Further investigation revealed that the phosphorane–(salen)CoI complex could also successfully catalyze the coupling of CO2 with aziridines under ambient conditions at a catalyst loading of 2.5 mol%.


 Please wait while we load your content...
Please wait while we load your content...