Conversion of C5 carbohydrates into furfural catalyzed by a Lewis acidic ionic liquid in renewable γ-valerolactone†
Abstract
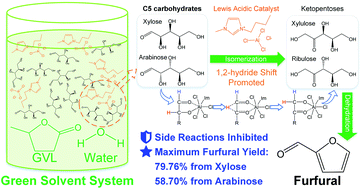
For the purpose of building a green reaction system to produce furfural (FF), the conversion of two important pentoses from hemicellulose, namely xylose and arabinose, was investigated in an aqueous reaction system including a Lewis acidic ionic liquid as a catalyst and renewable γ-valerolactone (GVL) as a co-solvent. The results showed that the introduction of GVL greatly improved the reactivity of pentose and inhibited the secondary decomposition reaction of FF compared to a pure-water reaction system. NMR analysis suggested that the composition of pentose conformers was greatly altered towards a reactive distribution. The highest FF yields were 79.76% (from xylose) and 58.70% (from arabinose), which were obtained at 140 °C. The influence of reaction parameters on pentose conversion was also studied. A comparison between different reaction conditions suggested that arabinose had less reactivity than xylose, leading to its lower conversion rate and FF yield. Furthermore, xylan and real biomass materials were tested in the proposed reaction system, and decent FF yields of up to 69.66% (from xylan) and 47.96% (from corn stalk) were obtained.


 Please wait while we load your content...
Please wait while we load your content...