Iridium(iii) homo- and heterogeneous catalysed hydrogen borrowing C–N bond formation†
Abstract
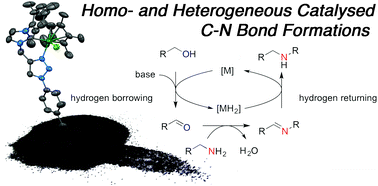
Pentamethylcyclopentadienyl iridium(III) complexes of bidentate carbene–triazole ligands were found to be excellent homogeneous catalysts for the hydrogen borrowing mediated coupling of alcohols with amines. The reactions are highly efficient, able to reach completion in under 6 h at 100 °C at low catalyst loadings of 0.5 mol%, and are environmentally benign, the only by-product is water. The Ir(III) catalysts promoted C–N bond formation across a range of alcohol and amine substrates, including biologically relevant motifs. Covalent attachment to a carbon black surface generated a well-defined “hybrid” heterogeneous catalyst which gave good conversion to products in the coupling reaction of aniline with benzyl alcohol, and could be recycled with no catalyst leeching. This represents the first report of a covalently linked heterogeneous iridium catalyst on carbon used for hydrogen borrowing. Turnover numbers (TON) for the heterogeneous were found to be significantly higher than the corresponding homogeneous reaction. To elucidate the homogeneous reaction mechanism, 1H NMR studies inconjuction with deuteration experiments allowed a mechanism to be postulated.


 Please wait while we load your content...
Please wait while we load your content...