Tandem cyclisation of vinyl radicals: a sustainable approach to indolines utilizing visible-light photoredox catalysis†
Abstract
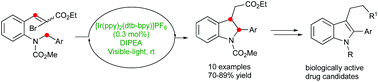
A tin-free method for the synthesis of substituted indolines has been developed generating vinyl radicals by visible-light-promoted photocatalysis in a reductive quenching cycle. This strategy offers a mild, robust, and high yielding pathway to a wide range of indolines containing diverse electronic substituents. The so obtained 2,3-disubstituted indolines serve as valuable precursors for the synthesis of biologically active molecules, which is demonstrated with the formal synthesis of tryptamines and furoindolines.


 Please wait while we load your content...
Please wait while we load your content...