Regio- and stereoselective ring-opening reaction of spiro-epoxyoxindoles with ammonia under catalyst-free conditions†
Abstract
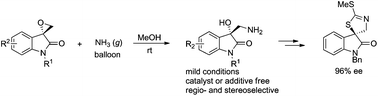
The regio- and stereoselective ring-opening of spiro-epoxyoxindoles with ammonia has been reported for the construction of 3-hydroxy-3-aminomethyloxindoles in high yields (up to 90%) and enantioselectivities (up to 99% ee) under mild and catalyst-free conditions. The reaction could be scaled up to the gram-scale and be used in the synthesis of analogues of dioxibrassinin and spirobrassinin.


 Please wait while we load your content...
Please wait while we load your content...