Low temperature aqueous phase hydrogenation of the light oxygenate fraction of bio-oil over supported ruthenium catalysts†
Abstract
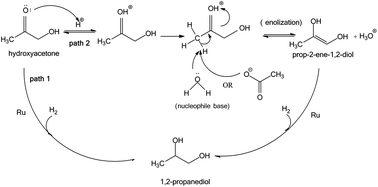
The low temperature hydrogenation of light oxygenates in the aqueous fraction of bio-oil (AFBO) was studied using both model compounds and a real bio-oil fraction with Ru/TiO2 and Ru/C catalysts in a continuous flow reactor. The hydrogenation of hydroxyacetone, acetic acid, and formic acid, in single and multi-compound aqueous mixtures, produced 1,2-propanediol, ethanol, and CO2 respectively, as the main products. The addition of acetic acid increased the rate of hydrogenation of hydroxyacetone by catalyzing the enolization of hydroxyacetone. The main reactions occurring during hydrogenation of the light oxygenates in the aqueous fraction of bio-oil included conversion of hydroxy-ketones into diols and hydrogenation of ketones and aldehydes into mono-alcohols. Differences in the product selectivity between the model carboxylic acids and AFBO were observed. High selectivity to ethanol was found when model-feed mixtures containing acetic acid were hydrogenated. In contrast, the ethanol selectivity for hydrotreating of the AFBO was 13 times lower than the ethanol selectivity for hydrotreating of acetic acid in water. A declining catalyst activity was observed when processing the AFBO.


 Please wait while we load your content...
Please wait while we load your content...