Amide-assisted radical strategy: metal-free direct fluorination of arenes in aqueous media†
Abstract
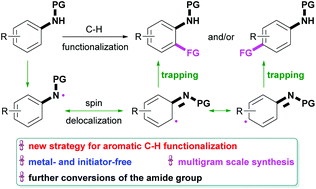
A metal- and initiator-free direct fluorination of arenes with the assistance of an amide group is developed. This reaction proceeded under simple aqueous conditions with good functional group tolerance and ortho–para selectivity, and is highly practical because it could be readily scaled up to a multigram-scale. At this stage, an exclusive mechanism could not be proposed, and several possibilities have been discussed. The possibility of the amide-assisted radical chain mechanism has been supported by experimental and computational investigations as well as a seminal work.


 Please wait while we load your content...
Please wait while we load your content...