Long-chain α–ω diols from renewable fatty acids via tandem olefin metathesis–ester hydrogenation†
Abstract
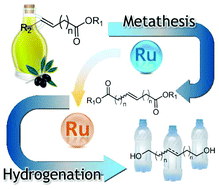
Long chain α–ω diols were readily accessed from renewable fatty acid methyl esters following an orthogonal tandem self-metathesis–ester hydrogenation protocol. By adding a base and a bidentate ligand, the metathesis catalysts were transformed in situ into efficient ester hydrogenation catalysts. The selectivity of the hydrogenation reaction was tuned towards the exclusive formation of either the unsaturated or the saturated diol by modifying the ligand/catalyst ratio. An orthogonal tandem cross-metathesis–ester hydrogenation reaction was also applied for the synthesis of a fragrance compound.


 Please wait while we load your content...
Please wait while we load your content...