Dimethyl sulfoxide as a mild oxidant in S–P(O) bond construction: simple and metal-free approaches to phosphinothioates†
Abstract
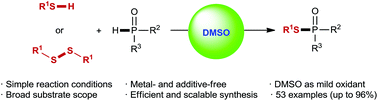
In the presence of dimethyl sulfoxide (DMSO) as a mild oxidant and reaction medium, a simple and efficient protocol has been developed for the preparation of phosphinothioates via oxidative dehydrogenative phosphorylation of thiols with P(O)H compounds. Additionally, a DMSO-mediated oxidative phosphorylation of disulfides is also demonstrated. Notably, these transformations occur efficiently without the help of any transition metal or additive. These reactions are easy to conduct and can be scaled-up, and various phosphinothioates are readily obtained in moderate to excellent yields with excellent chemoselectivity and good functional-group tolerance.


 Please wait while we load your content...
Please wait while we load your content...