Highly efficient synthesis of 2-mercaptobenzothiazole derivatives in water: metal sulfide–disulfide dynamic interchange reaction†
Abstract
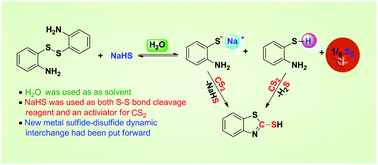
A convenient and efficient method for the synthesis of 2-mercaptobenzothiazoles from disulfide and CS2 mediated by a metal sulfide in water is described. This synthetic methodology could be used to prepare diverse 2-mercaptobenzothiazole derivatives in good to excellent yields. In this paper, the concept of metal sulfide–disulfide dynamic interchange reaction was put forward. Then the intermediates of the interchange reaction between NaHS and a disulfide were detected by LC-MS, which demonstrated that the S–S bond of the disulfide could be broken by the metal sulfide through the dynamic interchange reaction. In addition, NaHS was eventually transformed into sulfur S8 by the dynamic interchange reaction. Moreover, the underlying mechanism of 2-mercaptobenzothiazole formation is proposed, in which NaHS not only acts as an S–S bond cleaving agent but also as an activator of CS2. As a result, a novel synthetic route for the preparation of sulfur-containing heterocycles from a disulfide is developed.


 Please wait while we load your content...
Please wait while we load your content...