Olive leaf extract concentrated in hydroxytyrosol attenuates protein carbonylation and the formation of advanced glycation end products in a hepatic cell line (HepG2)
Abstract
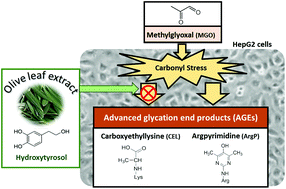
Glycation takes place both at the cellular level and at the extracellular matrix level and generates, consequently, advanced glycation end-products (AGEs) associated with chronic diseases and the aging process. Two olive leaf extracts concentrated in (i) oleuropein (OLE-A; 93.9 mg oleuropein g−1) and (ii) hydroxytyrosol (OLE-B; 54.5 mg hydroxytyrosol g−1) were evaluated according to their antiglycative and antioxidant capacity in vitro. OLE-B exerted the highest anti-AGE effect in different glycation models (IC50: 0.25–0.29 mg mL−1). OLE-B showed the highest antioxidant capacity and methylglyoxal-trapping capacity (IC50 0.16 mg mL−1). OLE-B showed a significant inhibitory effect against protein carbonylation (21%) and generation of argpyrimidine (26%) in a hepatocyte cellular carbonyl stress model evoked by methylglyoxal (MGO). OLE-B was further fractionated by solid phase-extraction, and the protective effect against protein carbonylation was only exerted by the fraction containing hydroxytyrosol. However, hydroxytyrosol standard, at the same concentration in the extract, inhibited the protein carbonylation below 10% but not significantly. The results indicate that the antiglycative activity of OLE in cells could be due to a synergic effect of hydroxytyrosol and other minor compounds with similar polarity. The research of the antiglycative activity in vivo could confirm these promising results and to propose OLE as a natural anti-AGE agent.


 Please wait while we load your content...
Please wait while we load your content...