Impact of the composition of the bacterial population and additional carbon source on the pathway and kinetics of degradation of endosulfan isomers
Abstract
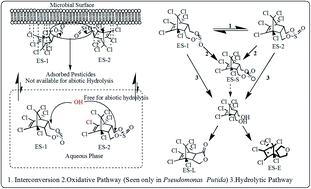
Abiotic and bacterial degradation is presented for the two isomers α- and β- of the organochlorine pesticide endosulfan, denoted as ES-1 and ES-2, respectively. Biodegradation studies were conducted with two indigenous species Pseudomonas putida (P. putida) and Rhodococcus sp. Both ES isomers rapidly hydrolyzed in water at pH ≥ 7 but the hydrolysis was inhibited in the presence of biomass. The pesticide partitioned onto the biomass making it unavailable for abiotic hydrolytic reaction. Spontaneous temperature dependent abiotic conversion of ES-2 to ES-1 was reported in the presence of dual air–water phases but was not observed in the abiotic aqueous phase. Biodegradation experiments with pure isomers showed a small amount of interconversion (∼5%) in either direction and ruled out any preferential interconversion of the ES-2 isomer to ES-1 or vice versa. Both the species were shown to degrade ES-2 at a higher rate compared to ES-1 which may lead to enrichment of ES-1 in agricultural fields in short-term following application of the pesticide. P. putida degraded both the ES isomers through oxidative and hydrolytic pathways while the Rhodococcus sp. used only the hydrolytic pathway. Since ES-S (product of the oxidative pathway) is orders of magnitude more toxic than the parent isomers, the short term toxicity of a field following the application of the pesticide may increase if the composition of the indigenous bacterial population is such that the oxidative pathway is preferred over the hydrolytic one. The presence of an additional carbon source increased the rates of degradation of both the isomers but the enhancement was greater for the degradation rate of ES-2 than ES-1.


 Please wait while we load your content...
Please wait while we load your content...