Crystal structure and chemical bonding in the mixed anion compound BaSF†
Abstract
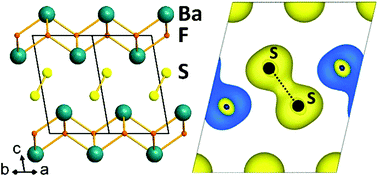
BaSF was synthesised by a solid state reaction at high temperature and its crystal structure was determined thanks to X-ray diffraction on a single crystal. This transparent yellow fluorochalcogenide has an intergrowth structure built from the stacking of fluorite type layers and sulfur layers. In BaSF sulfur atoms form dimers with interatomic distances as short as 2.1074(10) Å. DFT calculations confirm that this compound is a band insulator with the Fermi level lying in between the antibonding π* and σ* molecular orbitals of the sulfur dimers. Reflectance measurements show that the optical band gap of BaSF is about 2.7 eV in good agreement with the value found from DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...