Bis(silylenyl)-substituted ferrocene-stabilized η6-arene iron(0) complexes: synthesis, structure and catalytic application†
Abstract
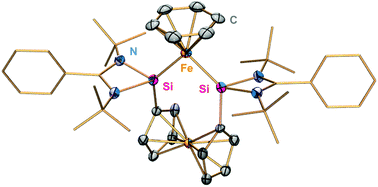
Reaction of FeX2(thf)n (X = Cl n = 1.5, Br n = 2) with the chelating 1,1′-bis(silylenyl)-substituted ferrocene ligand SiFcSiA (Fc = ferrocendiyl, Si = PhC(NtBu)2Si:) furnishes the corresponding dihalido Fe(II) complexes [(SiFcSi)FeX2] (X = Cl, 1 and X = Br, 2) in high yields. Reduction of the latter with an excess of KC8 in the presence of benzene and toluene leads to the unprecedented bis(silylene) stabilized Fe0 complexes [(SiFcSi)Fe-η6(C6H6)] 3 and [(SiFcSi)Fe-η6(C7H8)] 4, respectively. The 57Fe Mössbauer spectrum of 3 at 13 K exhibits parameters (σ = 0.3676 mm s−1; ΔEQ = 1.334 mm s−1) which are consistent with the presence of a pentacoordinated Fe0 atom in a pseudo trigonal–bipyramidal coordination environment, with two dative Si→Fe bonds and three coordination sites occupied by the η6-coordinated arene ligand. Results from DFT calculations, 57Fe Mössbauer parameters and the diamagnetic NMR spectra confirm the redox-innocent nature of these ligands and the zero oxidation state of the iron center. The catalytic ability of 3 was investigated with respect to ketone hydrogenation. In all cases, good to excellent yields to the corresponding alcohols were obtained at 50 °C and 50 bar H2 pressure. Electron-donating as well as -withdrawing substituents were tolerated with excellent to good yields. Conversions of bulkier ketones and unactivated aliphatic ketones lead merely to moderate yields. This represents the first example of a silylene-iron metal complex which has been utilized as a highly active precatalyst in the hydrogenation of ketones. The results underline the powerful ability of chelating bis(N-heterocyclic silylene) ligands acting as strong σ-donor ligands in stabilizing a new generation of low-valent, electron-rich transition metal complexes for catalytic transformations.


 Please wait while we load your content...
Please wait while we load your content...