Protein-templated gold nanoparticle synthesis: protein organization, controlled gold sequestration, and unexpected reaction products†
Abstract
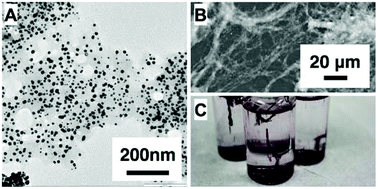
Emerging applications that exploit the properties of nanoparticles for biotechnology require that the nanoparticles be biocompatible or support biological recognition. These types of particles can be produced through syntheses that involve biologically relevant molecules (proteins or natural extracts, for example). Many of the protocols that rely on these molecules are performed without a clear understanding of the mechanism by which the materials are produced. We have investigated a previously described reaction in which gold nanoparticles are produced from the reaction of chloroauric acid and proteins in solution. We find that modifications to the starting conditions can alter the product from the expected solution-suspended colloids to a product where colloids are formed within a solid, fibrous protein structure. We have interrogated this synthesis, exploiting the change in products to better understand this reaction. We have evaluated the kinetics and products for 7 different proteins over a range of concentrations and temperatures. The key factor that controls the synthetic outcome (colloid or fiber) is the concentration of the protein relative to the gold concentration. We find that the observed fibrous structures are more likely to form at low protein concentrations and when hydrophilic proteins are used. An analysis of the reaction kinetics shows that AuNP formation occurs faster at lower protein (fiber-forming) concentrations than at higher protein (colloid-forming) concentrations. These results contradict traditional expectations for reaction kinetics and protein-fiber formation and are instructive of the manner in which proteins template gold nanoparticle production.


 Please wait while we load your content...
Please wait while we load your content...