The role of ligand redox non-innocence in ring-opening polymerization reactions catalysed by bis(imino)pyridine iron alkoxide complexes†
Abstract
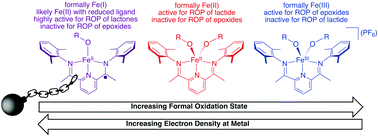
The reactivity of iron-based ring opening polymerization catalysts is compared when the catalyst is in three different oxidation states. Formally iron(I) monoalkoxide complexes 3a (p-methoxyphenoxide) and 3b (neopentoxide) supported by bis(imino)pyridine ligands were synthesized and investigated as catalysts for the ring opening polymerization and copolymerization of various monomers. For most monomers, 3a and 3b were superior catalysts compared to analogous, formally iron(II) and iron(III) complexes (1a/1b and 2a/2b, respectively) for the ring opening polymerization of various cyclic ester and cyclic carbonate monomers. Experimental and computational investigation into the electronic structures of 3a and 3b revealed that they are most accurately described as containing a high spin iron(II) center that is antiferromagnetically coupled to a singly reduced bis(imino)pyridine ligand. This electronic structure leads to increased electron density near the metal center without modulating the apparent metal oxidation state, which results in superior catalytic performance for the more highly reduced 3a and 3b compared to the increasingly more oxidized complexes (i.e.1a/1b and 2a/2b, respectively) in ring opening polymerization reactions. These findings have significant ramifications for the emerging field of redox-switchable polymerization catalysis.


 Please wait while we load your content...
Please wait while we load your content...