Dissimilar catalytic behavior of molecular or colloidal palladium systems with a new NHC ligand†
Abstract
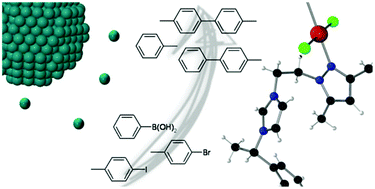
In this work, we describe the synthesis of a new N-heterocyclic carbene (NHC) ligand, derived from a hybrid pyrazole-imidazolium scaffold, namely 1-[2-(3,5-dimethylpyrazol-1-yl)ethyl]-3-((S)-1-phenylethyl)-3H-imidazol-2-ylidene (L). This ligand has been used as a stabilizer for the organometallic synthesis of palladium(0) nanoparticles (Pd NPs). L presents a better stabilizing effect than its pre-carbenic HLCl counterpart, allowing the formation of isolated Pd NPs while HLCl yields aggregated ones. Additionally, molecular Pd(II) coordination compounds of L and HLCl were synthesized and characterized to better understand the coordination modes of these ligands. Both molecular and colloidal Pd systems have been further tested in catalytic C–C coupling processes. Three different types of reactions have been observed depending on the catalytic system: (i) the Suzuki–Miyaura reaction takes place with Pd molecular complexes; (ii) a secondary reaction, the dehalogenation of the substrate, is always detected and (iii) the C–C homocoupling between two molecules of bromoarenes is observed with colloidal catalysts.


 Please wait while we load your content...
Please wait while we load your content...