Reactivity of the geminal phosphinoborane tBu2PCH2BPh2 towards alkynes, nitriles, and nitrilium triflates†
Abstract
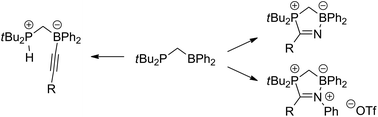
The reactivity of the geminal phosphinoborane tBu2PCH2BPh2 towards terminal alkynes, nitriles and nitrilium salts is investigated. Terminal alkynes react via C–H bond splitting (deprotonation) resulting in the formation of phosphonium borates. In contrast, both nitriles and nitrilium salts undergo addition reactions resulting in the formation of five-membered heterocycles. All compounds were characterized by multinuclear NMR spectroscopy, and single-crystal X-ray structure determinations. Insight into the reaction mechanisms was gained by DFT calculations.

 Please wait while we load your content...
Please wait while we load your content...