The mechanism for catalytic hydrosilylation by bis(imino)pyridine iron olefin complexes supported by broken symmetry density functional theory†
Abstract
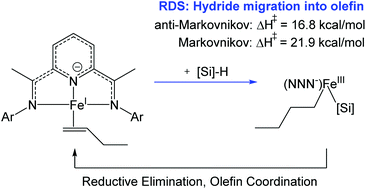
Density functional theory (DFT, B3LYP-D3 with implicit solvation in toluene) was used to investigate the mechanisms of olefin hydrosilylation catalyzed by PDI(Fe) (bis(imino)pyridine iron) complexes, where PDI = 2,6-(ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe)2(C5H3N) with Ar = 2,6-R2-C6H3. We find that the rate-determining step for hydrosilylation is hydride migration from Et3SiH onto the Fe-bound olefin to form (PDI)Fe(alkyl)(SiEt3). This differs from the mechanism for the Pt Karstedt catalyst in that there is no prior Si–H oxidative addition onto the Fe center. (PDI)Fe(alkyl)(SiEt3) then undergoes C–Si reductive elimination to form (PDI)Fe, which coordinates an olefin ligand to regenerate the resting state (PDI)Fe(olefin). In agreement with experimental observations, we found that anti-Markovnikov hydride migration has a 5.1 kcal mol−1 lower activation enthalpy than Markovnikov migration. This system has an unusual anti-ferromagnetic coupling between high spin electrons on the Fe center and the unpaired spin in the pi system of the non-innocent redox-active PDI ligand. To describe this with DFT, we used the “broken-symmetry” approach to establish the ground electronic and spin state of intermediates and transition states over the proposed catalytic cycles.
CMe)2(C5H3N) with Ar = 2,6-R2-C6H3. We find that the rate-determining step for hydrosilylation is hydride migration from Et3SiH onto the Fe-bound olefin to form (PDI)Fe(alkyl)(SiEt3). This differs from the mechanism for the Pt Karstedt catalyst in that there is no prior Si–H oxidative addition onto the Fe center. (PDI)Fe(alkyl)(SiEt3) then undergoes C–Si reductive elimination to form (PDI)Fe, which coordinates an olefin ligand to regenerate the resting state (PDI)Fe(olefin). In agreement with experimental observations, we found that anti-Markovnikov hydride migration has a 5.1 kcal mol−1 lower activation enthalpy than Markovnikov migration. This system has an unusual anti-ferromagnetic coupling between high spin electrons on the Fe center and the unpaired spin in the pi system of the non-innocent redox-active PDI ligand. To describe this with DFT, we used the “broken-symmetry” approach to establish the ground electronic and spin state of intermediates and transition states over the proposed catalytic cycles.


 Please wait while we load your content...
Please wait while we load your content...