A synthesis of novel expanded porphyrinoids: NiII-induced nitrile cyclization of dicyanovinylene-bis(meso-aryl)dipyrrin†
Abstract
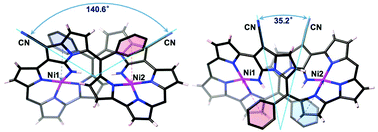
NiII-Metallation of dicyanovinylene-bis(meso-aryl)dipyrrin 1 under refluxed toulene conditions resulted in the formation of a bicyclic pyrrolizine ring to afford two isomeric bisNiII expanded porphyrinoid complexes 2a and 2b. Furthermore, acyclic NiII dipyrrin complex 3 was also isolated. The respective structures for 2a, 2b, and 3 were elucidated by X-ray diffraction analysis. Macrocycles 2a and 2b exhibited typical features of aromatic porphyrinoids showing their longest wavelength absorption bands in the near infrared region ranging from 1100 to 1600 nm.


 Please wait while we load your content...
Please wait while we load your content...