Isomeric effect on the properties of tetraplatinated porphyrins showing optimized phototoxicity for photodynamic therapy†
Abstract
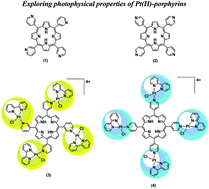
The design of new photosensitizers (PS) with improved properties is essential for the development of photodynamic therapy as an alternative therapeutic method. The conjugation of porphyrins, well known PS, with platinum(II) complexes, potent anticancer agents, may achieve new compounds with synergistic treatment effects and no side-effects. In this study, we synthesized para and meta isomers of free-base meso-tetra(pyridyl)porphyrins complexed to [PtCl(bipy)]+ units, and investigated their photophysics in solution and in lipid membrane vesicles, correlating with cell incorporation and viability results obtained from in vitro experiments using HeLa cells. Both porphyrins showed high singlet oxygen quantum yields and phototoxicity at the nanomolar scale, with green light irradiation (522 nm) and under very low light dose (1 J cm−2). The porphyrins showed LC50 values of 25 nM (meta) and 50 nM (para), which is remarkable for such mild conditions. Moreover, the phototoxicity difference between the isomers could be assigned to the higher amphiphilicity of the meta substituted porphyrin, which leads to improved lipid membrane interaction and cellular uptake compared to the para isomer.


 Please wait while we load your content...
Please wait while we load your content...