Imidazole/benzimidazole-modified cyclotriphosphazenes as highly selective fluorescent probes for Cu2+: synthesis, configurational isomers, and crystal structures†
Abstract
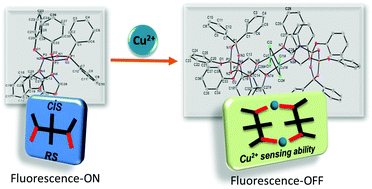
Configurational isomers (cis and trans) of imidazole- or benzimidazole-modified cyclotriphosphazenes (3a, 4a or 3b, 4b) were designed, synthesized and investigated as fluorescent probes for metal ions. The newly synthesized compounds were characterized by 1H and 31P NMR, and MALDI MS spectrometry. The configurations of geometric isomers were analyzed by X-ray crystallography and 31P NMR spectroscopy on addition of CSA. The photophysical behaviour and metal ion selectivity of the compounds were investigated by UV/vis and fluorescence spectroscopy. Among the examined 20 metal ions, the fluorescence emissions of the isomer mixtures were quenched by Cu2+ together with Fe2+, Fe3+, Zn2+ and Ni2+ ions, but each individual isomer (3a,b and 4a,b) exhibited an on–off-type fluorescence response with high selectivity towards only Cu2+ with a low limit of detection ranging from 1.27 μM to 2.04 μM. The complex stoichiometries of 3a,b and 4a,b with Cu2+ were determined as 1 : 1 (L/M) using the method of continuous variation (Job's plot) and density functional theory (DFT) calculations; moreover the complex formation of 4a with Cu2+ was unambiguously determined by X-ray crystallographic analysis that is consistent with the results obtained by the Job's plots as well as DFT.


 Please wait while we load your content...
Please wait while we load your content...