Silver(i)–organic frameworks constructed from silver(i) 3,6-pyrazyldiethynide and 3,8-1,10-phenanthrolyldiethynide complexes†
Abstract
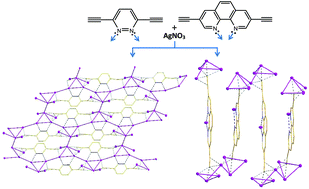
Two diynes bearing functional groups with different binding modes, 3,6-diethynylpyrazine (H2L1) and 3,8-diethynyl-1,10-phenanthroline (H2L2), were utilized as ligands to synthesize two new organometallic units, Ag2L1·3AgNO3 (1) and Ag2L2·6AgNO3 (2), in order to investigate the effect of the bridging and chelating modes of the ligands on the structures of networks constructed from silver-ethynide compounds. Structural studies show that in 1, silver-ethynide cluster units aggregate to form chair-like organometallic slides through Ag–N coordination bonds. These slides are linked through argentophilic interaction to generate novel 2D ladder-like layers, and are further bridged by nitrate anions to afford a 3D network in the solid state. It is observed that all the Ag ions in one layer interact to afford a 2D silver network. However, in 2, the silver-ethynide cluster units only interact to generate unique sine wave-like organometallic chains through argentophilic interaction, which are further connected by nitrate anions to form a 3D network. In the solid state, both 1 and 2 are luminescent at room temperature.


 Please wait while we load your content...
Please wait while we load your content...