FLP reduction and hydroboration of phenanthrene o-iminoquinones and α-diimines†
Abstract
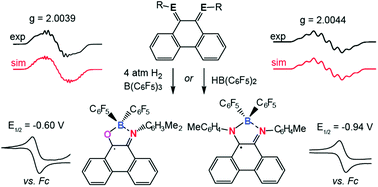
Redox active, or non-innocent, ligands containing O or N heteroatoms are frequently used in transition metal complexes, imparting unique catalytic properties, but have seen comparatively limited use in the chemistry of group 13 elements. In this article we report the frustrated Lewis pair (FLP) hydrogenation and hydroboration of an N-aryl-phenanthrene-o-iminoquinone and two N,N′-diaryl-phenanthrene α-diimines. These reactions exploit B(C6F5)3/H2, HB(C6F5)2 and H2BC6F5·SMe2 to give a series of derivatives including 1,3,2-oxaza- and diazaboroles and borocyclic radicals. The reaction pathways leading to these products are outlined and supported by DFT-calculations and experimental insight. The modular and unusual synthetic strategies described herein give access to new boroles as well as air-stable boron-containing radicals, thus extending the chemistry of redox active ligands in main group systems.


 Please wait while we load your content...
Please wait while we load your content...