A novel highly selective ligand for separation of actinides and lanthanides in the nuclear fuel cycle. Experimental verification of the theoretical prediction†
Abstract
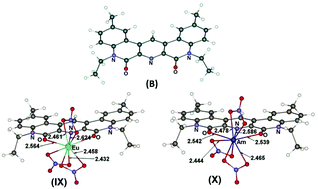
We have predicted earlier by DFT simulation that tridentate O,N,O-donor cyclic dilactams (B) belonging to the family of pyridine-2,6-dicarboxamides are much more selective and efficient extractants for the separation of lanthanides and actinides than open-structure pyridine-2,6-dicarboxamides due to the higher degree of “ligand preorganization”. In the present work, three new ligands of type (B) were synthesized. Extraction experiments showed that, in line with the data from DFT simulation, these ligands have 5–6-fold higher selectivity for the separation of an Am3+/Eu3+ pair and provide distribution coefficients D which are by three orders of magnitude higher than those for the related parent ligands with an open structure. Determination of the solvate numbers (SNs) for Eu3+ and Am3+ cations by slope analysis has shown that the stoichiometry of complexes, in the form of which these ions pass from the aqueous into the organic phase, depends to a considerable extent on the polarity of the organic solvent. Strongly polar solvents (ε > 20) extract these cations mainly in the form of 1 : 1 complexes LM(NO3)3 having according to the DFT simulation the largest dipole moments (μ = 18.6–19.7 D). The solvents of low polarity (ε ≤ 10) extract these cations mainly in the form of less polar 2 : 1 complexes L2M(NO3)3 (μ ≈ 1.6 D). For solvents of intermediate polarity fractional values of solvate numbers were obtained which indicates the coexistence of complexes LM(NO3)3 and L2M(NO3)3 in the organic phase.


 Please wait while we load your content...
Please wait while we load your content...