Quantification of Lewis acid induced Brønsted acidity of protogenic Lewis bases†
Abstract
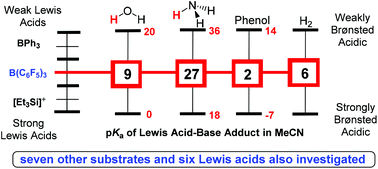
Proton transfer promoted by the coordination of protogenic Lewis bases to a Lewis acid is a critical step in catalytic transformations. Although the acidification of water upon coordination to a Lewis acid has been known for decades, no attempts have been made to correlate the Brønsted acidity of the coordinated water molecule with Lewis acid strength. To probe this effect, the pKa's (estimated error of 1.3 pKa units) in acetonitrile of ten protogenic Lewis bases coordinated to seven Lewis acids containing Lewis acidities varying 70 kcal mol−1, were computed. To quantify Lewis acid strength, the ability to transfer a hydride (hydride donor ability) from the respective main group hydride was used. Coordination of a Lewis acid to water increased the acidity of the bound water molecule between 20 and 50 pKa units. A linear correlation exhibiting a 2.6 pKa unit change of the Lewis acid–water adduct per ten kcal mol−1 change in hydride donor ability of the respective main group hydride was obtained. For the ten protogenic Lewis bases studied, the coordinated protogenic Lewis bases were acidified between 10 and 50 pKa units. On average, a ten kcal mol−1 change in hydride donor ability of the respective main group hydride resulted in about a 2.8 pKa unit change in the Brønsted acidity of the Lewis acid–Lewis base adducts. Since attempts to computationally investigate the pKa of main group dihydrogen complexes were unsuccessful, experimental determination of the first reported pKa of a main group dihydrogen complex is described. The pKa of H2-B(C6F5)3 was determined to be 5.8 ± 0.2 in acetonitrile.


 Please wait while we load your content...
Please wait while we load your content...