Coordination abilities of polycyano anions in the solid state: coordination geometries and d–d transition energies of mixed-ligand solvatochromic copper(ii) complexes with B(CN)4, C(CN)3, and N(CN)2 anions†
Abstract
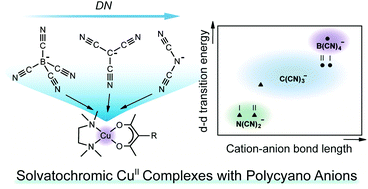
B(CN)4−, C(CN)3−, and N(CN)2− are highly versatile polycyano anions that produce various functional compounds. To investigate the coordination abilities of these anions in the solid state quantitatively, we synthesized mixed-ligand Cu(II) complexes: [Cu(R-acac)(tmen)X] (X = polycyano anion, R-acac = acetylacetonate or butyl-acetylacetonate, tmen = tetramethylethylenediamine). The coordination abilities of the anions, increasing in the order B(CN)4− < C(CN)3− < N(CN)2−, result in a decrease in the d–d transition energies of the complexes and the shortening of the axial coordination distance. The influence of crystal packing on the coordination geometries and d–d transition energies of the complexes was also demonstrated. The donor numbers of the anions were determined from the d–d transition energies in solution.


 Please wait while we load your content...
Please wait while we load your content...