Novel nickel(ii) complexes of sterically modified linear N4 ligands: effect of ligand stereoelectronic factors and solvent of coordination on nickel(ii) spin-state and catalytic alkane hydroxylation†
Abstract
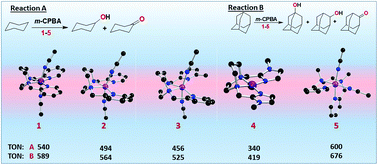
A series of Ni(II) complexes of the types [Ni(L)(CH3CN)2](BPh4)21–3, 5 and [Ni(L4)](BPh4)24, where L = N,N′-bis(2-pyrid-2-ylmethyl)-1,4-diazepane (L1), N-(6-methylpyrid-2-ylmethyl)-N′-(pyrid-2-ylmethyl)-1,4-diazepane (L2), N,N′-bis(6-methyl-2-pyridylmethyl)-1,4-diazepane (L3), N,N′-dimethyl-N,N′-bis(2-pyridylmethyl)ethylenediamine (L5) and L4 = N,N′-bis((1-methyl-1H-imidazole-2-yl)methyl)-1,4-diazepane, have been isolated and characterized. The complex cations of 1 and 4 possess, respectively, distorted octahedral and low-spin square planar coordination geometries in which nickel(II) is meridionally coordinated to all four nitrogen atoms of L1 and L4. DFT studies reveal that L5 with the ethylenediamine backbone coordinates in the cis-β mode in [Ni(L5)(CH3CN)2]2+5, but in the cis-α mode in [Ni(L5)(H2O)2]2+. Also, they illustrate the role of ligand donor atom type, diazacyclo backbone and steric hindrance to coordination of pyridyl nitrogen in conferring novel coordination geometries on Ni(II). All these complexes catalyse the oxidation of cyclohexane in the presence of m-CPBA as the oxidant up to 600 turnover numbers (TON) with relatively good alcohol selectivity (A/K, 5.6–7.2). Adamantane is oxidized to 1-adamantanol, 2-adamantanol and 2-adamantanone with high bond selectivity (3°/2°, 8.7–11.7). The incorporation of methyl substituent(s) on one (2) or both (3) of the pyridyl rings and the replacement of both the pyridylmethyl arms in 1 by imidazolylmethyl arms to give 4 decrease the catalytic efficiency. Interestingly, 5 with the cis-β mode of coordination provides two labile cis coordination sites for oxidant binding, leading to higher total TON and product/bond selectivity.


 Please wait while we load your content...
Please wait while we load your content...