Comparative solution equilibrium studies of antitumor ruthenium(η6-p-cymene) and rhodium(η5-C5Me5) complexes of 8-hydroxyquinolines†
Abstract
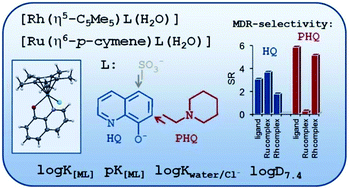
Complex formation processes of [Ru(η6-p-cymene)(H2O)3]+ and [Rh(η5-C5Me5)(H2O)3]+ organometallic cations with 8-hydroxyquinoline (HQ) ligands were studied in aqueous solution by the combined use of 1H NMR spectroscopy, UV-visible spectrophotometry and pH-potentiometry. Solution stability, chloride ion affinity and lipophilicity of the complexes were characterized together with the in vitro cytotoxicity against a pair of cancer cell lines, responsive and resistant to classic chemotherapy. The solid phase structure of the [Rh(η5-C5Me5)(8-quinolinolato)(Cl)] complex was characterized by single-crystal X-ray diffraction analysis. In addition to the unsubstituted HQ its 7-(1-piperidinylmethyl) (PHQ) and 5-sulfonate (HQS) derivatives were involved. PHQ has a significant preference for targeting multidrug resistant cancer cell lines, while HQS served as a water soluble model compound. The equilibrium studies revealed the formation of mono[M(L)(H2O)] complexes with prominently high solution stability, which predominate at physiological pH even in the micromolar concentration range, and the formation of mixed hydroxido [M(L)(OH)] complexes was characterized by relatively high pKa values (8.5–10.3). In comparison to the Rh(η5-C5Me5) species the complexation process with Ru(η6-p-cymene) is much slower, and both the pKa values and the H2O/Cl− co-ligand exchange constants are lower by 1–1.5 orders of magnitude. The stability order obtained for these organometallic complexes is as follows: HQS > HQ > PHQ. The cytotoxicity of the ligands and their Ru(η6-p-cymene) and Rh(η5-C5Me5) complexes was investigated against MES-SA (human uterine sarcoma) cell line and its multidrug resistant counterpart (MES-SA/Dx5). HQ and its complexes show similar cytotoxicity in both cell lines. In contrast, PHQ and its Rh(η5-C5Me5) complex are more potent against MES-SA/Dx5 cells, while this selectivity could not be observed for the Ru(η6-p-cymene) complex.


 Please wait while we load your content...
Please wait while we load your content...