Palladacycles of sulfated and selenated Schiff bases of ferrocene-carboxaldehyde as catalysts for O-arylation and Suzuki–Miyaura coupling†
Abstract
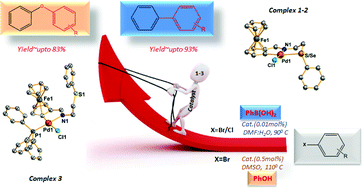
Schiff base ligands (L1: sulfated and L2: selenated) having a ferrocene core synthesized by reacting ferrocene-carboxaldehyde with 2-(phenylthio/seleno)ethylamine on treatment with Na2PdCl4 in the presence of NaOAc give cyclopalladated complexes [Pd(L1/L2-H)Cl] (1/2). Complex 1 of a sulfated Schiff base L1, on reacting with one equivalent of triphenylphosphine gives complex [Pd(L1-H)PPh3Cl] (3), formed due to cleavage of a Pd–S bond. With 2 such a reaction does not occur, as a Pd–Se bond being stronger than that of its sulfur analogue does not get cleaved. L1, L2 and their complexes 1–3 were authenticated with HR-MS, 1H, 13C{1H} and 77Se{1H} NMR spectroscopy. The single crystal structures of 1–3 were determined with X-ray diffraction. Palladium in all three complexes has nearly a square planar geometry. The Pd–S, Pd–Se and Pd–P bond distances are 2.4249(12), 2.5058(14) and 2.2445(17) Å respectively. The catalytic activity of complexes 1–3 was explored for O-arylation of phenol and Suzuki–Miyaura coupling (SMC) of phenylboronic acid with aryl bromides and chlorides. The optimum reaction time for SMC of ArBr is 3 h whereas for ArCl it is 6 h. The TON values of O-arylation catalyzed with complexes 1–3 are up to ∼170 (TOF, 28 h−1) and SMC ∼9300 (TOF, 3100 h−1) for the reaction time of the order of 3 and 6 h respectively. The catalytic process is somewhat more efficient with 2 (Pd bonded with a selenoether group), than 3, followed by 1.


 Please wait while we load your content...
Please wait while we load your content...