Synthesis, structure, and magnetic properties of new layered phosphate halides Sr2Cu5(PO4)4X2·8H2O (X = Cl, Br) with a crown-like building unit†
Abstract
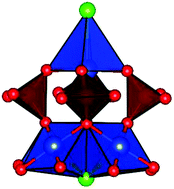
Two new compounds Sr2Cu5(PO4)4X2·8H2O (X = Cl and Br) are synthesized by a conventional hydrothermal method. Sr2Cu5(PO4)4Cl2·8H2O crystallizes in the tetragonal system with a space group of P4212, while Sr2Cu5(PO4)4Br2·8H2O crystallizes in the space group P4/nmm, which are found to have a similar framework of layered structure, in which the crown-like {Cu5(PO4)4X2} building units connect to each other forming a 2D corrugated sheet with vacancies, while the Sr2+ cations are located along the vacancies. The spin lattice of two compounds built by Cu2+ ions shows a new type of corrugated square. Magnetic measurements confirmed that both Sr2Cu5(PO4)4X2·8H2O (X = Cl and Br) exhibit antiferromagnetic ordering at low temperatures. A fit of theoretical model shows exchange interaction J = −25.62 K for the Cl-analogue and J/kB = −26.47 K for the Br-analogue.


 Please wait while we load your content...
Please wait while we load your content...