Al-Based coordination polymer nanotubes: simple preparation, post-modification and application in Fe3+ ions sensing†
Abstract
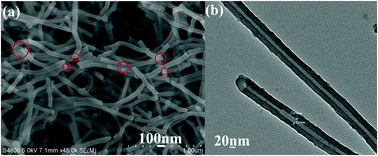
Aluminum-based coordination polymers, MIL-110(Al) nanotubes, were successfully prepared from a mixed solution of methanol and ethanol with the volume ratio of 10 : 10 at room temperature in the absence of any template or surfactant; AlCl3 and sodium 1,3,5-benzenetricarboxylate (Na3BTC) were employed as the initial reactants. The composition, structure, and morphology of the as-obtained nanotubes were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive spectrometry (EDS), thermogravimetric analysis (TGA), and inductively coupled plasma atomic emission spectroscopy (ICP-AES). It was found that the initial reaction medium played a crucial role in the formation of nanotubes. More importantly, MIL-110(Al) nanotubes could be fabricated into lanthanide-functionalized luminescent materials by a simple post-treatment process. The as-obtained luminescent materials could emit red, green, and white light, which have potential applications in the fields of sensing, biomedicine, labeling, and color display. Furthermore, the PL of the as-obtained luminescent materials could be selectively quenched by Fe3+ ions, which can be used as probes for the detection of Fe3+ ions.


 Please wait while we load your content...
Please wait while we load your content...