Controlled synthesis of cyclosiloxanes by NHC-catalyzed hydrolytic oxidation of dihydrosilanes†
Abstract
Hydrolytic oxidation of various hydrosilanes in acetonitrile and in the absence of organic solvents catalyzed by an N-heterocyclic carbene organocatalysis is described. The NHC organocatalyst exhibited a very high activity with only 0.1 mol% loading of the catalyst in acetonitrile for aryl-substituted dihydrosilanes to produce hydrogen gas and cyclosiloxanes almost quantitatively in several minutes. The calculated TOF (15 000 h−1) of this organocatalyst is comparable to those of precious metal-based heterogeneous catalysts and much superior to those of the existing homogeneous metal catalysts. The catalytic reaction selectively yielded cyclosiloxanes in high yield without the contamination of silanols. Furthermore, the catalytic reaction can also be furnished under solvent-free conditions at elevated temperatures with 2.5 mol% loading of the NHC in 5–12 hours.
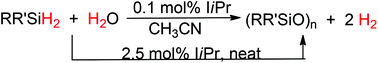
- This article is part of the themed collections: Dalton Transactions Inorganic Symposia and Silicon Chemistry: Discoveries and Advances


 Please wait while we load your content...
Please wait while we load your content...