Structural transformations of layered structures constructed from Cu(ii)–chloranilate monomer compounds†
Abstract
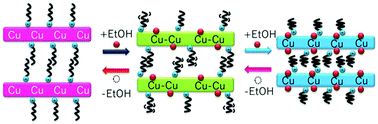
Hydrogen-bond supported intercalation compounds constructed from three types of two-dimensional layers and common guests, {(Hha)2[Cu(CA)2(EtOH)2]}n (1), {(Hha)2[Cu(CA)2(EtOH)]}n (2), and {(Hha)2[Cu(CA)2]}n (3) (ha = hexylamine), have been synthesized and characterized. The hexylaminium cations are introduced between the anionic layers of the metal–chloranilate by not only electrostatic interactions but also hydrogen bonding interactions. These compounds show reversible EtOH adsorption and desorption accompanied by a structural transformation. With a change in the interaction between the mononuclear complexes of the host layer, the interaction between the host layer and the intercalated guest cations also changes. Moreover, the mobility and flexibility of the intercalated Hha+ cations enable the rearrangement of the mononuclear complexes while maintaining their layer structure. Thus, these compounds have flexibilities both in the inter- and intra-layers.


 Please wait while we load your content...
Please wait while we load your content...