Fluoride complexation by bidentate silicon Lewis acids†
Abstract
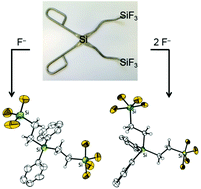
Diethynyldiphenylsilane (1) and divinyldiphenylsilane (2) were functionalized by hydrosilylation reactions with HSiMe2Cl, HSiMeCl2 and HSiCl3. Fluorination of the resulting compounds generates bidentate open-chain Lewis acids of increasing acidity. All semi-flexible [Ph2Si(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHSiFnMe3−n)2 (n = 1, 2, 3)] and flexible [Ph2Si(CH2–CH2SiFnMe3−n)2 (n = 1, 2, 3)] bidentate Lewis acids were obtained in good to excellent yields. The different fluoride ion complexation behavior was explored in detail by multinuclear (low temperature) NMR spectroscopy. The Lewis acidic bidentate molecules as well as the resulting mono- and bissilicates were completely characterized by NMR spectroscopy, mass spectrometry and in part by elemental analysis and X-ray diffraction experiments.
CHSiFnMe3−n)2 (n = 1, 2, 3)] and flexible [Ph2Si(CH2–CH2SiFnMe3−n)2 (n = 1, 2, 3)] bidentate Lewis acids were obtained in good to excellent yields. The different fluoride ion complexation behavior was explored in detail by multinuclear (low temperature) NMR spectroscopy. The Lewis acidic bidentate molecules as well as the resulting mono- and bissilicates were completely characterized by NMR spectroscopy, mass spectrometry and in part by elemental analysis and X-ray diffraction experiments.


 Please wait while we load your content...
Please wait while we load your content...