New, potentially chelating NHC ligands; synthesis, complexation studies, and preliminary catalytic evaluation†
Abstract
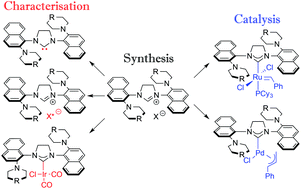
Two new N-heterocyclic carbene (NHC) ligands bearing 2-morpholino and 2-piperidinyl naphthyl wingtips were synthesised (2-SIMorNap and 2-SIPipNap). Nuclear magnetic resonance studies, in conjunction with crystal structures and derivatisation of the NHC salts using a chiral counteranion, revealed that the ligand wingtips are oriented anti with respect to each other. From the free carbene, palladium, ruthenium and iridium complexes were prepared. NHC–iridium dicarbonyl complexes were made in order to extract the TEP values for these ligands. The study showed that these NHC ligands are more electron-donating than normal, aryl-substituted NHCs. The palladium complexes were tested in representative Suzuki–Miyaura cross-coupling reactions and compared to the state of the art systems. Ruthenium-catalysed ring-closing metathesis with these ligands was also performed. It was found that Grubbs’ 2nd generation catalyst incorporating 2-SIPipNap did not initiate at room temperature and required heating for RCM to occur.


 Please wait while we load your content...
Please wait while we load your content...