Single-molecule magnet behaviour in a tetranuclear DyIII complex formed from a novel tetrazine-centered hydrazone Schiff base ligand†
Abstract
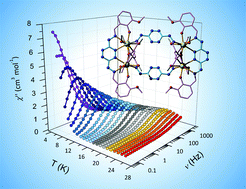
Two analogous tetranuclear lanthanide complexes have been synthesized with the general formula [Ln4(vht)4(MeOH)8](NO3)4·aMeOH·bH2O, where H2vht = (3,6-bis(vanillidenehydrazinyl)-1,2,4,5-tetrazine) and Ln = DyIII (1), GdIII (2). These complexes are characterized by several techniques; including single-crystal X-ray diffraction, SQUID magnetometry and single-crystal micro-SQUID hysteresis loop measurements. Elucidation of the crystal structure of the complexes shows that the lanthanide ions are bridged by a tetrazine ring, a rare bridging moiety for lanthanide ions. Magnetic studies reveal that both 1 and 2 exhibit weak ferromagnetic exchange interactions between Ln ions, and 1 displaying Single-Molecule Magnet (SMM) behaviour with a magnetisation reversal barrier of Ueff = 158 K (τ0 = 1.06 × 10−7 s).
- This article is part of the themed collection: CSC100: Celebrating Canadian Chemistry


 Please wait while we load your content...
Please wait while we load your content...