A thiourea-functionalized metal–organic macrocycle for the catalysis of Michael additions and prominent size-selective effect†
Abstract
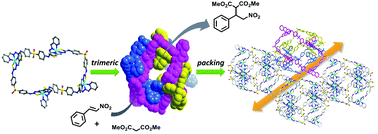
A discrete tetranuclear thiourea-based metal–organic macrocycle (MOM) with a large size was constructed by a well-designed organic ligand and nickel(II) ions via self-assembly. Incorporating thiourea groups as hydrogen-bond donors into a metal–organic complex system leads to a new approach for synthesizing functionalized heterogeneous catalysts, as this not only introduces coordination sites serving as chelators, but also overcomes the issues of self-association via intermolecular H-bonding, often occurring in homogeneous systems. The packing structure of this material formed a confined environment suitable for the access of substrate molecules dragged by the strong hydrogen-bond interactions from the thiourea groups, thus achieving a high catalytic performance in Michael additions of nitrostyrenes to nitroalkanes, with remarkable yields and size-selectivity in heterogeneous phase. Moreover, a comparison of the IR spectrum of Ni–SPT with the spectra of dimethyl malonate- and β-nitrostyrene-impregnated Ni–SPT indicated that both substrate molecules, β-nitrostyrene and dimethyl malonate, were able to access the cavity of the trimeric subunit.


 Please wait while we load your content...
Please wait while we load your content...