Tridentate Lewis-acids based on triphenylsilane†
Abstract
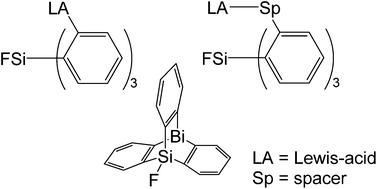
Several derivatives of the propeller-shaped ortho-substituted triphenylsilanes, carrying metal- or silicon-based acceptor groups, are reported. They were synthesized starting from tris(2-bromophenyl)fluorosilane, tris(2-vinylphenyl)fluorosilane and tris(2-ethynylphenyl)fluorosilane to generate a scope of Lewis-acidic molecules with different cavities. An improved synthetic protocol for donor-free tris(2-lithiophenyl)silanes is described. First attempts in host–guest chemistry to probe the binding between a threefold alane-functionalised ortho-substituted triphenylsilane and a tridentate Lewis-basic guest molecule are presented. The synthesis and a first molecular structure determination in the crystalline state of a bismasilatriptycene is reported.


 Please wait while we load your content...
Please wait while we load your content...